Abstract
Background Despite the increased effectiveness of combination drug therapy, multiple myeloma (MM) remains incurable, and patients eventually succumb to relapsed disease. With each subsequent line of therapy, relapsed/refractory MM (RRMM) becomes more challenging to treat as high-quality responses become harder to achieve. Therefore, there remains a significant unmet need for new combination regimens that leverage drugs with novel mechanisms of action to improve outcomes in MM. REGN5458 is a bispecific antibody capable of binding to B-cell maturation antigen (BCMA) on MM cells and CD3 on T cells, inducing targeted T-cell-mediated cytotoxicity of MM cells. Results from a Phase I dose escalation study of REGN5458 monotherapy in patients with RRMM showed early, deep, and durable responses with a manageable safety and tolerability profile (NCT03761108; Zonder, et al. EHA 2022. Abstract S189). Among patients treated at the 200-800 mg dose levels, the response rate was 75%. Cytokine release syndrome was reported in 38% of patients, and the severity was mostly Grade 1 with no Grade ≥3 events. Given the encouraging early efficacy and limited overlapping toxicity of REGN5458, it is reasonable to explore the potential benefit of combining REGN5458 with other anti-myeloma agents, with the aim of improving the depth and duration of responses.
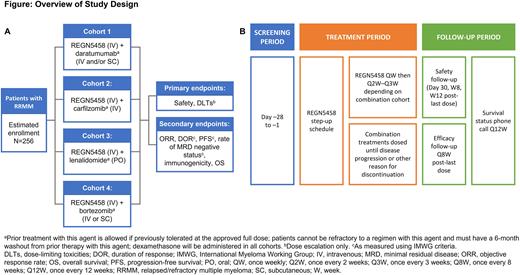
Study Design and Methods This global, Phase Ib, open-label, multi-cohort study (NCT05137054) is designed to assess the safety, tolerability, and efficacy of REGN5458 in combination with other cancer treatments in patients with RRMM. There are 4 cohorts (Figure panel A), where REGN5458 will be combined with daratumumab plus dexamethasone (cohort 1); carfilzomib plus dexamethasone (cohort 2); lenalidomide plus dexamethasone (cohort 3); and bortezomib plus dexamethasone (cohort 4). Eligible patients cannot be refractory to the cohort-specific combination treatment, but prior exposure is permitted provided at least a 6-month washout period has elapsed.
The study will include a dose-finding portion to select an appropriate REGN5458 dose, followed by a dose-expansion portion for each cohort. REGN5458 will be administered intravenously on a weekly basis starting with a step-up dosing schedule. After 15-16 weeks, the dosing frequency will be reduced to every 2-3 weeks depending on the cohort. The other cancer treatments will be administered at the approved doses. All regimens will be given until disease progression or any other reason for discontinuation (Figure panel B).
Estimated total enrollment is up to 256 patients. The study will accrue patients with RRMM that have progressed after ≥3 lines of therapy, or ≥2 lines of therapy and either prior exposure to at least 1 anti-CD38 antibody, 1 immunomodulatory drug (IMiD), and 1 proteasome inhibitor (PI) or double refractory to 1 PI and 1 IMiD. Eligible patients must be aged ≥18 years, have an Eastern Cooperative Oncology Group performance status ≤1, and have adequate liver and kidney function. Key exclusion criteria are the presence of MM brain lesions or meningeal involvement, and previous treatment with a BCMA-directed bispecific antibody (BCMA-directed antibody-drug conjugate is permitted) or chimeric antigen receptor T cell therapy.
The primary endpoint is safety and tolerability of the REGN5458 combinations, in terms of dose-limiting toxicities (dose-finding portion only) and treatment-emergent adverse events for each cohort. Secondary endpoints are objective response rate, duration of response, progression-free survival, minimal residual disease status, pharmacokinetics, incidence of anti-drug antibodies, and overall survival. There is no formal statistical hypothesis for this study. The results will be reported in a descriptive manner. Exploratory analyses will evaluate measures of immune cell function in the bone marrow and peripheral blood that may be modulated by the combination regimens. Biomarkers of particular interest in the bone marrow include plasma and immune cell phenotypes, BCMA expression, and soluble BCMA. This study is actively recruiting patients at sites across the United States, Spain, France, and Greece.
Disclosures
Rodríguez Otero:Amgen: Speakers Bureau; GSK: Consultancy, Speakers Bureau; Pfizer: Consultancy; Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; BMS: Consultancy; BMS-Celgene: Speakers Bureau; Regeneron: Speakers Bureau. Joseph:Janssen: Research Funding; GSK: Research Funding; BMS: Research Funding. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Lee:Pfizer: Consultancy; Regeneron: Research Funding; Janssen: Research Funding; Takeda Pharmaceuticals: Consultancy, Research Funding; Oncopetides: Consultancy; Amgen: Research Funding; Immunitas Therapeutics: Consultancy; Sanofi: Consultancy; Legend Biotech: Consultancy; Genentech: Consultancy; Karyopharm: Consultancy; Monte Rosa Therapeutics: Consultancy; GSK: Consultancy, Research Funding. Leleu:Amgen: Honoraria; Pfizer: Honoraria; Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; BMS: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria. Manier:Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Dimopoulos:Amgen: Honoraria; Takeda: Honoraria; BMS: Honoraria; Beigene: Honoraria; Janssen: Honoraria. Mateos:Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides, Seagen: Honoraria; Takeda: Honoraria, Other: advisory board participation; Regeneron: Honoraria, Other: advisory board participation; Roche: Honoraria, Other: advisory board participation; GSK: Honoraria, Other: advisory board participation; Pfizer: Honoraria, Other: advisory board participation; Sanofi: Honoraria, Other: advisory board participation; Bristol-Myers Squibb: Honoraria, Other: advisory board participation; Janssen: Honoraria, Other: advisory board participation; Amgen: Honoraria, Other: advisory board participation. Oriol:Janssen: Consultancy; Sanofi: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; GlaxoSmithKline: Consultancy, Speakers Bureau. Bumma:Sanofi, Genzyme: Other: Ad Board, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Ad Board; Amgen: Consultancy, Speakers Bureau. Gong:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Roy:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Rodriguez Lorenc:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kroog:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Sarkaria:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company.
OffLabel Disclosure:
Off-Label Drug Purpose: The abstract describes the study design and methods of a global, Phase Ib, open-label, multi-cohort study exploring REGN5458 in combination with other cancer treatments in patients with relapsed/refractory multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal